论文标题:targeting muc1-c suppresses bcl2a1 in triple-negative breast cancer
期刊:
作者:
masayuki hiraki, takahiro maeda, neha mehrotra, caining jin, maroof alam, audrey bouillez, tsuyoshi hata, ashujit tagde, amy keating, surender kharbanda, harpal singh & donald kufe
发表时间:2018/05/12
数字识别码:10.1038/s41392-018-0013-x
原文链接:
微信链接:
原文作者:masayuki hiraki, takahiro maeda, neha mehrotra, caining jin, maroof alam, audrey bouillez, tsuyoshi hata, ashujit tagde, amy keating, surender kharbanda, harpal singh & donald kufe
美国哈佛大学医学院dana-farber癌症研究所donald william kufe教授在施普林格自然与川大华西医院生物治疗国家重点实验室联合主办的signal transduction and targeted therapy(sttt)上发表研究论文,发现靶向对abt-737耐药的三阴性乳腺癌细胞中的muc1-c可以抑制bcl2a1并诱导死亡,这很可能成为新的治疗三阴性乳腺癌策略。

三阴性乳腺癌(triple negative breast cancer,tnbc)是指雌激素受体(er)、孕激素受体(pr)和人表皮生长因子受体2(her-2)均为阴性的一种乳腺癌。三阴性乳腺癌占所有乳腺癌病理类型的15%-23.8%,三阴性乳腺癌具有侵袭力强、恶性程度高等特点,三阴性乳腺癌目前尚没有有效的治疗方式,对其它类型的乳腺癌有效的内分泌治疗和分子靶向(如靶向her-2的曲妥珠单抗)对tnbcs均无效。目前,三阴性乳腺癌主要依靠化疗,但治疗后愈很差,且无复发生存率和总生存率均相对较低。b细胞淋巴瘤2相关蛋白a1(bcl2a1)是bcl-2抗凋亡蛋白家族的一员,其癌症细胞对化疗药物和靶向药物的耐药相关。bcl2a1通过阻断细胞死亡发挥癌基因的功能。目前,在三阴性乳腺癌中发现了bcl2a1的过表达,然而,目前还没有有效的药物能靶向过度表达的bcl2a1,从而治疗癌症。
黏蛋白1(muc1)是一种在约90%的三阴性乳腺癌中过表达的异二聚体蛋白。muc1跨膜c-末端(muc1-c)起到癌蛋白的作用,它可与多种激酶和效应物进行相互作用,影响多条信号通路。例如, muc1-c能激活与炎症相关的tgf-β激活激酶1(tak1),进而影响 tak1→ikk→nf-κb p65通路。muc1-c胞内结构域直接与nf-κb p65相互作用,促进nf-κb p65靶基因的活化,其中包括muc1,进而形成自诱导循环,增加muc1-c的表达。目前有研究表明,muc1-c还能通过nf-κb p65依赖性机制增加抗凋亡bcl-x蛋白的表达,此外muc1-c在三阴性乳腺癌细胞对bcl-2抑制剂的氧化应激反应中稳定mcl-1。但目前的研究还未发现muc1-c信号通路与bcl2a1之间有关系。
为研究两者的关系,donald kufe教授课题组在针对三阴性乳腺癌细胞mda-mb-468和bt-20的研究发现, muc1-c诱导bcl2a1表达(图1)。
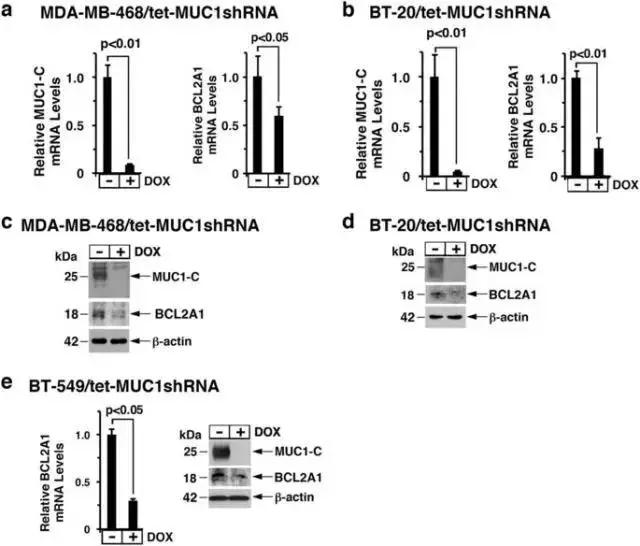
图1 downregulation of muc1-c decreases bcl2a1 expression
随后,研究人员使用靶向cqc基序并抑制muc1-c同源二聚化的go-203肽(图2a)进行研究,go-203肽已被纳入用于递送到肿瘤细胞中的纳米颗粒聚合物中(go-203 / np),研究表明靶向muc1-c能抑制bcl2a1的表达。
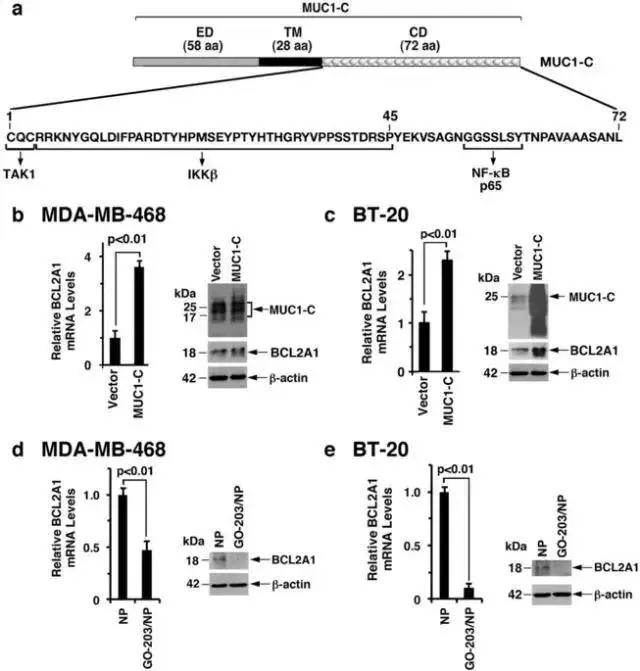
图2 muc1-c drives bcl2a1 expression
进一步的研究发现,muc1-c是通过nf-κb p65介导的机制促进bcl2a1的转录(图3)。
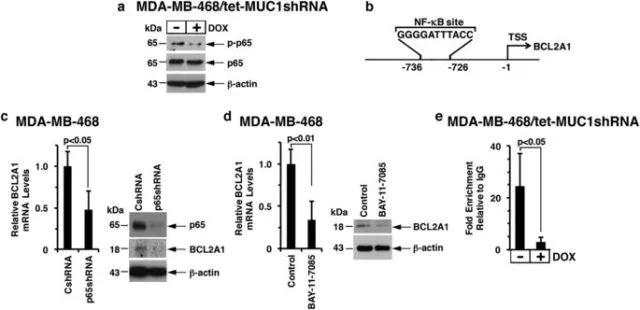
图3 muc1-c→nf-κb p65 signaling induces bcl2a1 expression
随后,研究人员发现,三阴性乳腺癌对abt-737和abt-263这类靶向bcl的药物耐药与muc1-c→nf-κb→bcl2a1途径的激活有关。而使用靶向muc1-c的go-203 / np能有效抑制abt-737耐药三阴性乳腺癌中的bcl2a1,从而治疗三阴性乳腺癌(图4,图5)。
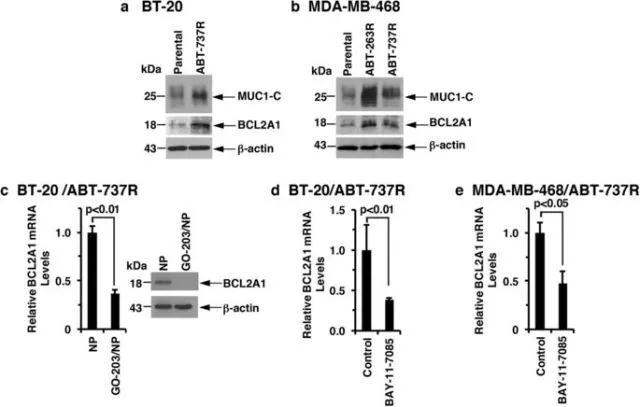
图4 muc1-c→nf-κb signaling upregulates bcl2a1 in abt-737-resistant cells
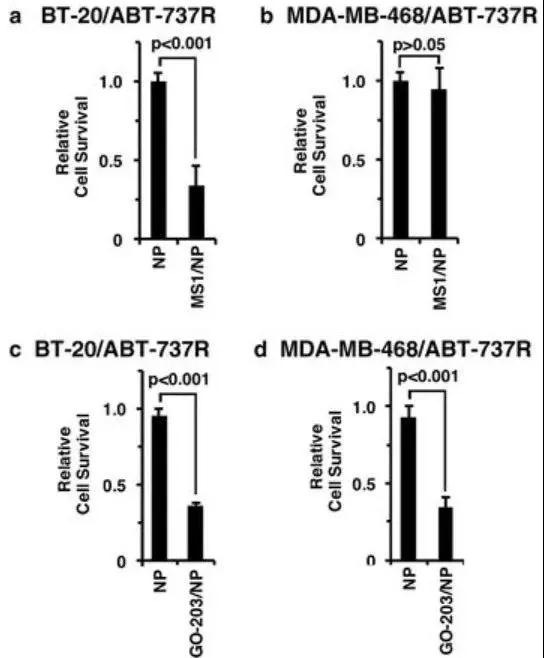
图5 targeting muc1-c is effective against abt-resistant cells with bcl2a1 overexpression
综上,本研究发现,muc1-c通过nf-κb p65依赖性机制在tnbc细胞中诱导bcl2a1的表达。实验显示muc1-c→nf-κb p65→bcl2a1通路对于用针对bcl-2家族的其他成员的药物(例如abt-737)的治疗反应极为敏感。进一步的研究表明靶向muc1-c能有效下调对abt-737耐药的tnbc细胞中的bcl2a1,进而治疗三阴性乳腺癌(图6)。
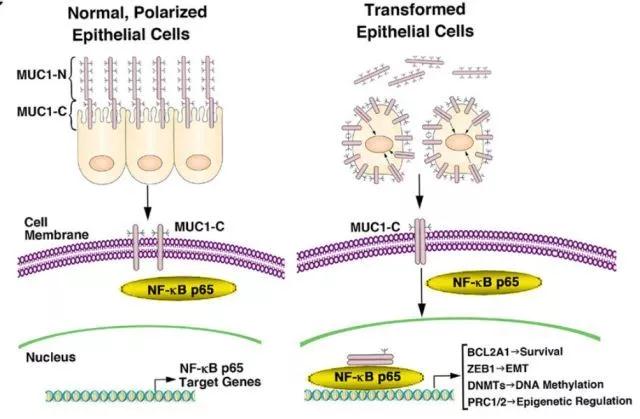
图6 schema depicting the function of the muc1-c→nf-κb p65 pathway in integrating the induction of bcl2a1 expression with the emt program and epigenetic regulation
摘要:
b-cell lymphoma 2-related protein a1 (bcl2a1) is a member of the bcl-2 family of anti-apoptotic proteins that confers resistance to treatment with anti-cancer drugs; however, there are presently no agents that target bcl2a1. the muc1-c oncoprotein is aberrantly expressed in triple-negative breast cancer (tnbc) cells, induces the epithelial–mesenchymal transition (emt) and promotes anti-cancer drug resistance. the present study demonstrates that targeting muc1-c genetically and pharmacologically in tnbc cells results in the downregulation of bcl2a1 expression. the results show that muc1-c activates the bcl2a1 gene by an nf-κb p65-mediated mechanism, linking this pathway with the induction of emt. the mcl-1 anti-apoptotic protein is also of importance for the survival of tnbc cells and is an attractive target for drug development. we found that inhibiting mcl-1 with the highly specific ms1 peptide results in the activation of the muc1-c→nf-κb→bcl2a1 pathway. in addition, selection of tnbc cells for resistance to abt-737, which inhibits bcl-2, bcl-xl and bcl-w but not mcl-1 or bcl2a1, is associated with the upregulation of muc1-c and bcl2a1 expression. targeting muc1-c in abt-737-resistant tnbc cells suppresses bcl2a1 and induces death, which is of potential therapeutic importance. these findings indicate that muc1-c is a target for the treatment of tnbcs unresponsive to agents that inhibit anti-apoptotic members of the bcl-2 family.
阅读论文全文请访问:
期刊介绍:
signal transduction and targeted therapy ()(issn 2059-3635 (online), issn 2095-9907 (print), cn 51-1758/r) is a new open access journal, which aims to accomplish timely publication of the latest discoveries and progress in both basic science and clinical research related to signal transduction and targeted therapy. it will include research on major human diseases, particularly on cancer.
(来源:科学网)
特别声明:本文转载仅仅是出于传播信息的需要,并不意味着代表本网站观点或证实其内容的真实性;如其他媒体、网站或个人从本网站转载使用,须保留本网站注明的“来源”,并自负米乐app官网下载的版权等法律责任;作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。